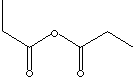
PROPIONIC ANHYDRIDE
PRODUCT IDENTIFICATION
123-62-6
TOXICITY
SMILES
CLASSIFICATION
EXTRA NOTES
UN2496 [Corrosive]
PHYSICAL AND CHEMICAL PROPERTIES
clear liquid
167 C
1.01
285 C
REFRACTIVE INDEX
1.403 - 1.405
63 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Local: Propionic anhydride is a clear liquid with an unpleasant odour. It hydrates with water producing corrosive propionic acid. It is miscible in most organic solvents and decomposes with alcohol. Propionic anhydride used as an intermediate to produce dyes, pharmaceuticals, agrochemicals and other organic compounds.
GENERAL DESCRIPTION OF ANHYDRIDE: Anhydride is a compound formed by the abstraction of a molecule of water, H2O, from a substance. The term acid anhydride is restricted sometime to the anhydride formed especially from an acid by dehydration or one that revert to the original substance upon hydration. In case of bimolecular, it can be composed of two molecules of the corresponding acid. The term mixed anhydride is an acid anhydride composed of two different acids. Examples are adenosine triphosphate or an aminoacyl adenylate. The anhydrides of bases are oxides.
Anhydrides of inorganic acids are usually oxides of nonmetallic elements. Carbon dioxide (CO2) is the anhydride of carbonic acid, dinitrogen pentoxide (N2O5) is the anhydride of nitric acid, sodium oxide is an anhydride of sodium hydroxide, phosphorus pentoxide (P2O5) is the anhydride of phosphoric acid, and sulfur trioxide (SO3) is the anhydride of sulfuric acid. An acid anhydride forms an acid; a base anhydride forms a base. Sulfur trioxide (acid anhydride) reacts with water to form sulfuric acid (an acid product). Calcium oxide (an base anhydride) reacts with water to form calcium hydroxide (a base product).
Organic anhydrides contain the carbonyl group (CO). Organic anhydrides are formed by the condensation of original acids. Lactone, an internal cyclic monoester, is an anhydride derived from the hydroxyl and carboxyl radicals. In organic chemistry, most anhydride compounds are derived from corresponding carboxylic acids. Carboxylic anhydrides, general formula (RCO)2O, are the dehydration product of two carboxylic acid molecules. The name of carboxylic anhydride is given first from the original acid, followed by the separate word "anhydride". [CH3(CH2)2CO]2O is butanoic anhydride, CH3COOCOCH2CH3 is ethanoic propanoic anhydride (or acetic propionic anhydride). Anhydrides are more reactive than the parent acids. Anhydrides are typically not target molecules, but rather they are used as intermediates for the synthesis of other organic members such as esters and amides for the industrial applications include dyes, pharmaceuticals, pesticides, plastics, fibers, curing agents, plasticizers and many others. The reactivity of carboxylic acid derivatives are in order of acyl halides > anhydrides >> esters �� acids >> amides. Anhydrides react with alcohols to form esters; acetic anhydride [(CH3CO)2O] reacts with ethanol (C2H5OH) to form ethyl acetate (CH3COOC2H5) used as a common solvent. Anhydrides also react with ammonia and primary or secondary amines to form amides. Anhydrides react with water to form their corresponding acids.
APPEARANCE
clear liquid
98.0% min
PROPIONIC ACID
2.0% max
INDIVIDUAL IMPURITY
0.5% max
20 max (Pt/Co scale)
SAFETY INFORMATION
GHS Hazard Statements : H314-H227 Causes severe skin burns and eye damage. Combustible liquid.
Precautionary Statements : P210-P260-P303+P361+P353-P305+P351+P338-P405-P501A Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Do not breathe dust/fume/gas/mist/vapours/spray. IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Store locked up. Dispose of contents/container in accordance with local/regional/national/international regulations.
PRICE INFORMATION